 The original article on “Surfactants” (a contraction of the three words “Surface Active Agents”) was written in the fall of 2015i. Although the general topic of surfactants has not changed in nearly 6 years, several new chemistries have surfaced, and there wasn’t nearly as much emphasis on bio-based or green chemistry back then, as there is today. In addition, if you are new to coatings you should also be looking at ancillary markets such as personal care and cosmetics. Prospector has an excellent expert in Belinda Carli. See her article entitled “How to Select Natural Surfactants”ii. A natural surfactant has to have both the head and tail groups to come from truly natural sources. Personal care surfactants often have the same chemistry as paint surfactants, but perhaps different names or slightly different functions. Another article that pulls it all together is “breaking that tension with surfactants”iii
The original article on “Surfactants” (a contraction of the three words “Surface Active Agents”) was written in the fall of 2015i. Although the general topic of surfactants has not changed in nearly 6 years, several new chemistries have surfaced, and there wasn’t nearly as much emphasis on bio-based or green chemistry back then, as there is today. In addition, if you are new to coatings you should also be looking at ancillary markets such as personal care and cosmetics. Prospector has an excellent expert in Belinda Carli. See her article entitled “How to Select Natural Surfactants”ii. A natural surfactant has to have both the head and tail groups to come from truly natural sources. Personal care surfactants often have the same chemistry as paint surfactants, but perhaps different names or slightly different functions. Another article that pulls it all together is “breaking that tension with surfactants”iii
Thirty percent of global respondents are willing to pay a premium for products that deliver on social accountability claims (Nielsen, 2020). Bio-based surfactants are designated by the new EN17035. The chemical industry is now able to use a transparent definition and division which also enables to communicate in a comparable way to the end-user. Companies such as BASF, Ethox, Solvay and Locus Fermentation Solutions, among many others, are producing more bio surfactants.
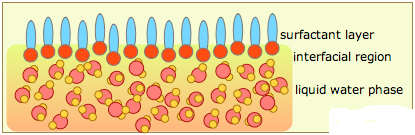
Surfactants are materials that lower the surface tension (or interfacial tension) between two liquids or between a liquid and a solid. In the general sense, any material that affects the interfacial surface tension can be considered a surfactant, but in the practical sense, surfactants may act as wetting agents, emulsifiers, foaming agents, and dispersants, among others.
Surfactants play an important role as dispersing, emulsifying, cleaning, wetting, foaming and anti-foaming agents in many practical applications and products, including: paints, emulsions adhesives, inks, biocides (sanitizers), shampoos, toothpastes, firefighting (foams), detergents, insecticides, deinking of recycled papers, ski waxes, spermicides (nonoxynol-9). This is an article about paint, which means surfactants that are used in paint, emulsions, wetting agents, and in many items used in paint that are dispersed or emulsified.
The dynamics of surfactant adsorption is of great importance for practical applications such as in emulsifying or coating processes as well as foaming, where bubbles or drops are rapidly generated and need to be stabilized. As the interface is created, the adsorption is hindered by the diffusion of the surfactant to the interface, which can result in the kinetics being limited. These energy barriers can be due to steric or electrostatic repulsions; steric repulsions form the basis of how dispersants work. Surface rheology of surfactant layers, is important to the stability of foams and emulsions.
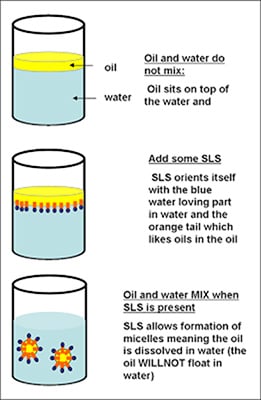 In the bulk aqueous phase, surfactants form masses, such as micelles, where the hydrophobic tails form the core and the hydrophilic heads are immersed in the surrounding liquid. Other types of structures can also be formed, such as spherical micelles or lipid bilayers. The shape of the molecules depends on the balance in size between hydrophilic head and hydrophobic tail. A measure of this is the HLB, Hydrophilic-lipophilic Balance. Higher HLB surfactants (>10) are hydrophilic (“water loving”) and form O/W (Oil-in-water) emulsions. Lipophilic surfactants possess low HLB values (1-10) and form W/O (water-in-oil) emulsions. Dish detergents, surfactants for emulsion polymerization, and the following example (SLS = Sodium Lauryl Sulfate) are high HLB surfactants.
In the bulk aqueous phase, surfactants form masses, such as micelles, where the hydrophobic tails form the core and the hydrophilic heads are immersed in the surrounding liquid. Other types of structures can also be formed, such as spherical micelles or lipid bilayers. The shape of the molecules depends on the balance in size between hydrophilic head and hydrophobic tail. A measure of this is the HLB, Hydrophilic-lipophilic Balance. Higher HLB surfactants (>10) are hydrophilic (“water loving”) and form O/W (Oil-in-water) emulsions. Lipophilic surfactants possess low HLB values (1-10) and form W/O (water-in-oil) emulsions. Dish detergents, surfactants for emulsion polymerization, and the following example (SLS = Sodium Lauryl Sulfate) are high HLB surfactants.
Most surfactants’ “tails” are fairly similar, consisting of a hydrocarbon chain, which can be branched, linear, or aromatic. Fluorosurfactants have fluorocarbon chains. Siloxane surfactants have siloxane chains. Recent advances in surfactant technology have seen the development of mixed chains or/and complex structures. One example of mixed chain/complex structures is N,N-dimethyldodecylamine oxide (DDAO) and sodium decyl-, sodium dodecyl- and sodium tetra-decylsulfate (abbreviated as SDeS, SDS and STS, respectively).
There are 4 types of surfactants with a brief review of each as follows. These classifications are based upon the composition of the polarity of the head group: nonionic, anionic, cationic, amphoteric.

A non-ionic surfactant has no charge groups in its head. The head of an ionic surfactant carries a net charge. If the charge is negative, the surfactant is more specifically called anionic; if the charge is positive, it is called cationic. If a surfactant contains a head with two oppositely charged groups, it is termed zwitterionic. Commonly encountered surfactants of each type are listed as follows. A complete compendium can be found on www.ULProspector.com.
Nonionic surfactant
Many long chain alcohols exhibit some surfactant properties. Some examples of non-ionic surfactants include:
| Trade name | Structure/name | Applications |
| Triton™ X-100 | Polyoxyethylene glycol octylphenol ethers: C8H17–(C6H4)–(O-C2H4)1–25–OH | Wetting agent – coatings |
| Nonoxynol-9 | Polyoxyethylene glycol alkylphenol ethers: C9H19–(C6H4)–(O-C2H4)1–25–OH | Spermacide |
| Polysorbate | Polyoxyethylene glycol sorbitan alkyl esters | Food ingredient |
| Span® | Sorbitan alkyl esters | Polishes, cleaners, fragrance carriers |
| Poloxamers, Tergitol™, Antarox® | Block copolymers of polyethylene glycol and polypropylene glycol | Various |
Anionic surfactant
Anionic surfactants contain anionic functional groups at their head, such as sulfonate, phosphate, sulfate and carboxylates. Alkyl sulfates include ammonium lauryl sulfate, sodium lauryl and the related alkyl-ether sulfates sodium laureth sulfate, also known as sodium lauryl ether sulfate (SLES), and sodium myreth sulfate. These are the most common surfactants and comprise the alkyl carboxylates (soaps), such as sodium stearate. The stearates comprise >50% of the global usage of surfactants. Many of these find utilization in emulsion polymerization. Other anionic surfactants include dioctyl sodium sulfosuccinate (DOSS), linear alkylbenzene sulfonates (LABs), as well as alkyl-aryl ether phosphates.
| Trade name | Structure/name | Applications |
| Pentex 99 | Dioctyl sodium sulfosuccinate (DOSS) | Wetting agent – coatings, toothpaste |
| PFOS | Perfluorooctanesulfonate (PFOS) | Scotchguard™, Skydrol™ |
| Calsoft® | Linear alkylbenzene sulfonates | Laundry detergents, dishwasher detergents |
| Texapon® | Sodium lauryl ether sulfate | Shampoos, bath products |
| Darvan® | Lignosulfonate | Concrete plasticizer, plasterboard, DMSO |
| N/A | Sodium stearate | Handsoap, HI&I products |
Cationic surfactant
Cationic surfactants are comprised of a positively charged head. Most of cationic surfactants find use as anti-microbials, anti-fungals, etc. in household, institutional and industrial cleaners (Benzalkonium chloride (BAC), Cetylpyridinium chloride (CPC), Benzethonium chloride (BZT)). The cationic nature of the surfactants is not typically consistent with the world of non-ionic and anionic charges, and they disrupt cell membranes of bacteria and viruses. Permanently charged quaternary ammonium cations include: Alkyltrimethylammonium salts: cetyl trimethylammonium bromide (CTAB) and cetyl trimethylammonium chloride (CTAC).
Zwitterionic surfactants
 Zwitterionic (amphoteric) surfactants have both cationic and anionic centers attached to the same molecule. The anionic part can be variable and include sulfonates, as in the sultaines CHAPS (3-[(3-Cholamidopropyl)dimethylammonio]-1-propanesulfonate). Betaines such as cocamidopropyl betaine have a carboxylate with the ammonium. The cationic part is based on primary, secondary, or tertiary amines or quaternary ammonium cations. Zwitterionic surfactants are often sensitive to pH and will behave as anionic or cationic based on pH. Fast dry (“coacervation”) latex traffic paints are based on this concept, with a drop in pH triggering the latex in the paint to coagulate.
Zwitterionic (amphoteric) surfactants have both cationic and anionic centers attached to the same molecule. The anionic part can be variable and include sulfonates, as in the sultaines CHAPS (3-[(3-Cholamidopropyl)dimethylammonio]-1-propanesulfonate). Betaines such as cocamidopropyl betaine have a carboxylate with the ammonium. The cationic part is based on primary, secondary, or tertiary amines or quaternary ammonium cations. Zwitterionic surfactants are often sensitive to pH and will behave as anionic or cationic based on pH. Fast dry (“coacervation”) latex traffic paints are based on this concept, with a drop in pH triggering the latex in the paint to coagulate.
Resources
[i] Surface Active Agents (Surfactants): Types and Applications (ulprospector.com)
[ii] How to select natural surfactants for personal care products (ulprospector.com)
[iii] Surfactants in paints: how they work and current market trends | Prospector (ulprospector.com)
Explore more than 48,000 materials for the paint and coatings industry with Prospector! Search now to find what you need!
The views, opinions and technical analyses presented here are those of the author or advertiser, and are not necessarily those of ULProspector.com or UL Solutions. The appearance of this content in the UL Prospector Knowledge Center does not constitute an endorsement by UL Solutions or its affiliates.
All content is subject to copyright and may not be reproduced without prior authorization from UL Solutions or the content author.
The content has been made available for informational and educational purposes only. While the editors of this site may verify the accuracy of its content from time to time, we assume no responsibility for errors made by the author, editorial staff or any other contributor.
UL Solutions does not make any representations or warranties with respect to the accuracy, applicability, fitness or completeness of the content. UL Solutions does not warrant the performance, effectiveness or applicability of sites listed or linked to in any content.



It was an illustrating article.
thank you.
This is Jennifer from Bangladesh. We are manufacturers of textile chemical so we need non-ionic surface active agents. Every month we need 40 tons. Presently we are buying from BASF named Luteno The o8. Do you have any counter product like this. If so then do let me know soon.
Best regards,
Jennifer Kamal.
Jennifer, I write general articles to help people learn. I am a consultant and even if I did work for a surfactant company, it wouldn’t be ethical for me to recommend something. I suggest that if you are looking for off-sets, you contact your local distributors who carry surfactants other than BASF’s and ask them to ask their principals what they recommend as offsets to the BASF product.
Marc, thanks for your prompt reply. I am glad to meet such an ethical person. As I understood that you are a consultant so I would like to offer you to become my company’s consultant.
i need to multifunctional agent surfactant and non-emulsifier for acidizing stimulation
I am not sure I understand your need. If you are asking about a surfactant stable in acidic conditions, etc. I suggest that you contact your local global surfactant suppliers and work with them to a solution.
In what units is the HLB expressed?
HLB does not have units.
Hi Marc,
Very informative article, I enjoyed reading while having my coffee break. Please post some more article regarding how to fix fish eye problems in latex paint if possible. Appreciate!
Thanks
Congratulations very explicit. When you talk about interface. What is it?
In the physical sciences, an interface is a surface forming a common boundary among two different phases of matter, such as an insoluble solid and a liquid, two immiscible liquids, a liquid and an insoluble gas or a liquid and vacuum
Sanjay, this is not an easy subject to address in a comment and there are a lot of good articles on coatings defects and their cause and prevention (including in the archives of Prospector). There are several issues to consider. Are the fisheyes apparent on the substrate on to which the coating will be applied, or is it on a surrogate substrate? E.g., people use a Leneta 1B or 2B chart and drawdown coatings, but the shear from a wire bar or a blade is far less than from spraying, and a sealed card would not really be a good substrate if the actual substrate were wood for example.
If you feel that the film thickness, application shear, etc. are all OK, then you can look at the defoamer. Is the fisheye problem immediate and dissipates over a few days? Do you hear age the paint? The likelihood of paint being manufactured and used within days is near zero, so if the issue goes away in a short time, I wouldn’t be concerned. And you should be checking the long-term stability and efficacy of your defoamers.
But generally, what you describe is an imbalance of surfactants; meaning typical (dispersant, wetting agent, etc.). Sometimes a defoamer can be too strong (silicone) not dispersed sufficiently, or needs to be put in the grind vs. letdown. Are you seeing fisheyes or crawling? Crawling would be a coatings whose surface tension differs too greatly from the substrate and cannot wet it out.
Good luck!
Dear Mr Hirsch,
Please, let me introduce a brief comment into your very interesting paper.
You mentioned Scotchgard products as materials manufactured with PFOS.
Since 2003, 3M Company has replaced PFOS based products by C4 chemicals, I mean, perfluoro butane derivates.
This kind of surfactants are Novec 4430, Novec 4432.
Thanks for your attention
Regards
Thank you for your advise, I will do H.A.S test to see if aging would help the issue.
Thanks again.
Thank you very much. Nice article, clear and precise
Hi, interesting, I would like to be advised which surface active agent is good in recycling waste engine oil, ratio of sulfuric acid to surfactant.
Kind regards,
Mbugua KAMAU.
can you please suggest any anionic or non-ionic surfactant that does not foam in water.
Could you pls, suggest non ionic surfactant for anti foam for potato washing company in food industry.
Thanks Doctor.. Such an informative article. I am looking for a 250-300 deg. C stable surfactant which can also act as an emulsifier. The application is welding.
we are india base manufacturers of all non ionic surfactantes manufacturers and exports we supply of same quality of products many type indian textiles axulary chemical
very brifing article!
would u please give me some guide how to make waterless car wash wax solution
for my poor country. be thankyou.
sir,
thx for you given a good article for my learning carrier. sir i am working in agrochemical company as a research officer pl suggest me how to selection of the surfectant for my new pesticides formulation as like (SC . EC. WDG. Etc).
Thanks for the informative article. Can you suggest surfectant for food processing areas, baby bottle cleaners and fruits and vegetable washing? Please suggest surfactant family names to check with local suppliers.
thanks
Sheraz
curious your thoughts on surfactants on painted surfaces… could certain surfactants break down the paint? not sure if it is non-anionic, anionic, cationic or amphoteric… not sure it maters if being applied to a painted surface.
could surfactants break down the painted surface changing its appearance?
Hi; i need surfactant to micro emulsion 30% water with Diesel oil, can you develop one and sell to me?
Thank you,
ST
Dear sir can you give best formulation for liquid laundry detergent.
formulation with full chemical details so that I can understand.
Hi Kuldeep,
first you need to understand your need.
1. what kind of formulation
2. what concentration
3. active stability
4. formulation physical stability
5. mode of action of active
6. last but not least, cost of formulation.
once you identify your requirement, than you go for the selection of formulation process and screening.
screening is purely based on the experise you have in surfactant chemistry.
this can be developed over a period of time.
I hope my respone has served you well.
I NEED AN ARTICLE WHICH CONTAINS
MICROBIAL PRODUCTION OF SURFACE ACTIVE AGENTS
Dear sir
Highly informative topic.
Could you provide some information about surfactants for making hydrocarbon (c5) resin dispersion which has application in natural rubber latex adhesive.
Thanking you
We are manufacturer of textile chemicals in india. Our requirement of eco friendly non ionic surfactant it Is water miscible,wetting and rewetting properties,oily soil ,graphite,grease remover for polyeaster pretreatment and also use in dyeing.
I apologize in that I just am seeing this. I do not manufacture anything, I am a consultant. In the development of product and applications, most suppliers are willing to help you to develop a solution and may even do the work for you in their labs. Therefore, I suggest that you contact your local suppliers and ask for their recommendations.
Please discuss with your local supplier of surfactants for their recommendation
Most food grade defoamers (and some are Halal and Kosher) are silicone emulsions in water. You can find manufacturers by searching on UL Prospector: https://www.ulprospector.com/
By the nature of what a surfactant is, anything that is added to pure water, will cause foam of some type. There are many that produce little foam, or the foam dissipates quickly. A local supplier of surfactants (and ideally also of defoamers) can recommend the best product to use.
Metal working fluids? That high temperature is a little difficult. You really should be looking at oils that survive in that temperature range. At high temps, depending on agitation, they can be self-emulsifying.
I want to understand the right wetting agent to disperse sodium naphthalene formaldehyde powder in water to have better dispersion, request you to suggest the right wetting agent or surfactant.
Hi. The article really isn’t about car waxes. I suggest that you perform an internet search on the subject or you can find manufacturers by searching on UL Prospector: https://www.ulprospector.com/
Most food grade defoamers (and some are Halal and Kosher) are silicone emulsions in water. You can find manufacturers by searching on UL Prospector: https://www.ulprospector.com/
There are certainly surfactants that are either high or low pH that could affect surface appearance. Also some chemistries. If this is a concern, a sample of the paint (chip) can be removed and sent to a laboratory for analytical testing.
I am sorry, but these articles are to educate and there are other means to obtain this information. You can find manufacturers by searching on UL Prospector: https://www.ulprospector.com/ You can also participate on LinkedIn in a Surfactant group where perhaps you can engage a consultant who you can pay to provide a solution
I suggest that you perform a literature search using any one of the internet search engines
You can find manufacturers by searching on UL Prospector: https://www.ulprospector.com/ Most all raw material suppliers have technical support groups who help customers with this sort of question.
You can find manufacturers by searching on UL Prospector: https://www.ulprospector.com/ Most all raw material suppliers have technical support groups who help customers with this sort of question.
You can find manufacturers by searching on UL Prospector: https://www.ulprospector.com/ Most all raw material suppliers have technical support groups who help customers with this sort of question.
Hi have a additive to emulsify 30% water to 70% Diesel oil. Appearance of micro emulsion will be milky white.
Could you please suggest me, some of the Non-ionic surfactants which will useful in dispersion of nonparticles while adding with polymers.
I am not sure I understand your question. English is not your first language? I think you are asking for the dispersion of nanoparticles in a polymer?
I suggest you look at any manufacturer of dispersants and search for nano materials. As an example, use the database from UL Prospector and start with BYK Altana. Once on their site, search for nano particle dispersion. They are just one of many suppliers. I just used them as an example.
can i get a few examples of amphiphilic surfactant with lower HLB?
Please contact one of the numerous surfactant suppliers which can be found on UL to answer this.
Hello Mr Iyer.
With reference to your post on additive to emulsify water and diesel, could you confirm you have this additive for sale.
I would be interested… Thank you.
S Suriyanarayanan
Hello marc. What is the best surfactants for making a little oil to mix with water? I planned using this as an organic insecticide for my crops.
It depends on several things. If this is for personal use and you don’t mind that the surfactant I recommend isn’t organic, then I would suggest that the leasp expensive and easier would be to use dish soap. If you want truly organic, I could recommend organic (soy based) surfactants, or you can find these on UL. It may seem as if you provided enough information to respond with a definitive answer, but you really didn’t. There are lots of oils with a whole range of viscosities, solubilities, etc.
There are several KP articles on natural surfactants for personal care use if that is your goal.
I am still learning about this science. Thanks for expanding my knowledge.
I manufacture the only all temperature ski waxes in the world. We recently have been banned from using fluoro within the wax. I am looking for an advance Surfactant to replace the FC.
I mentored under Dr. Tim Donnelly out of Davis
I recommend that you contact suppliers of waxy additives such as Shamrock, MPI, BYK Altana, etc. Also, silicone manufacturers. They will be of the most help to you.
hi, I am a research student and looking for how much paracetamol extract from industrial wastewater. So, can you please suggest me non-ionic surfactant which is best to extract paracetamol? thank you
Your best bet is to contact surfactant companies and ask them. This article is for general information and what you are asking is very specific.
Hi, I want modifile beeswax for surface funiture. I think can mix beeswax with some surace active agent. What do you think? What kind of agent?
You would be best served to contact a local large surfactant manufacturer that is local to you. Ask them for help. There are many grades of beeswax.
Nice and tnx as well it really helps me alot
Hello Girish
Please send us your web site
My mail address is : [email protected]
Brds
Giorgio Koutsoukos
ZEUS CHEMICALS- Greece
I’m not sure who you replied to. I don’t know who Girish Patel is. My email address is [email protected]
Hi need your advice,
can u recommend brand of water based anti-foam.
There are many defoamers that are water-based. You have to provide a lot more information for me to give you a useful response. Or use prospector knowledge center.
Dear Sir/madam,
We M/S October Co Ltd Situated at Riyadh Saudi Arabia. we are leading company for Road construction in our region. We need SURFACTANT COMPOUND to make FOAM for DUST SUPPRESSION to use in our project.
Kindly send us your Competitive offer as earlier is possible.
Regards
MD K ALAM
Mob.0536047536
[email protected]
You misunderstand the purpose of Prospector. I can’t provide you with a competitive offer, even if I sold surfactants. This is strictly for educational purposes.
It’s not as simple as just suggesting that you use a surfactant. A lot will depend on the hardness of water that you use, the temperature it is sprayed at, and if you’re looking for a foam that will be persistent or just wet the dust.
I suggest that you contact your local surfactant suppliers because that will also effect what is recommended-what is commercially available in your location.
Hi, I wondered if you could recommend which type of surfactant I would need to use to mix a Sodium salt solution with a wax, say a C14 or C16 chain length?
It is good
Thankyou
Thank you
It depends. I hate saying that, but it’s not straightforward. In your case, it appears you work for a personal care company, so I think the type of surfactant that you need to mix with the sodium solution with the wax is also dependent on the temperature and also the hardness of the water. Assuming that you’re using DI water, temperature still becomes a big part with cloud point. You would be best to contact your local supplier, and make sure that you have all of the production parameters before you call.
can you tell me the application of surfactant as solubilizing agent
Here is one.
In Vitro Cell Dev Biol Anim. 2011 Oct;47(9):631-9. doi: 10.1007/s11626-011-9449-9. Epub 2011 Sep 5.
Evaluation of surfactants as solubilizing agents in microsomal metabolism reactions with lipophilic substrates.
Randall K1, Cheng SW, Kotchevar AT.
Author information
Abstract
Solubilizing agents are routinely added when investigating the biotransformation of lipophilic substrates using hepatic microsomes. For highly lipophilic compounds, the concentration of solvent or surfactant necessary for dissolution can be detrimental to enzyme activity. This study evaluates the effect of 12 surfactants on microsomal metabolism and the ability of the same surfactants to improve the aqueous solubility of the pentabrominated diphenyl ether BDE-100, a lipophilic environmental contaminant previously found to be recalcitrant to in vitro metabolism. Of the surfactants investigated, Cremophor EL and Tween 80 displayed the best combination of increased BDE-100 solubility and minimal inhibition of microsomal metabolism. However, a comparison of the in vitro metabolism products of BDE-100 in the presence of the two surfactants revealed varying amounts of metabolites depending on the surfactant used.
PMID: 21898118 DOI: 10.1007/s11626-011-9449-9
Hi Marc Hirsch. I am working in a pharmaceutical company. this is regarding povidone iodine cleansing solution. we tried lot of surfactants but we are unable to stabilize the product. we are not able to identify Povidone Iodine content while using SLES. is there any other Surfactants that can be used to stabilize this products. should we use nonionic surfactants along with povidone iodine
Yes, it is suggested that you use non-ionic surfactant.
A povidone-iodine medicated dressing
JC Lawrence – Journal of Wound Care, 1998 – magonlinelibrary.com
… and causes only transient staining to materials. In iodophors, Iodine is in loose
combination with a non-ionic surfactant; in povidone-iodine this is polyvinyl-pyrrolidone
(povidone). A simple test shows that povidone-iodine readily …
Clinical applications of povidone-iodine as a topical antimicrobial
S Ripa, N Bruno, RF Reder, R Casillis… – Handbook of topical …, 2003 – books.google.com
… CURlTY®, Kendall) is an aqueous solution that comes formulated with a variety of detergents,
emulsifiers, surfactants, and moisturizers … nonoxynol-9) 0.5% PVP-I 0.3% PVP-I, surfactant
(nonoxyl-9) 10% PVP-I, surfactant (nonoxyl-10 … Povidone-Iodine as a Topical Antimicrobial 83 …
Hi Marc, thanks for the article – it’s really interesting!
I’ve recently been looking into a side project to try and address the issue of starchy foods boiling over during cooking. Online there are a lot of suggestions to add a small amount of oil to the water as it acts as a surfactant and increases the surface tension of the starchy layer so that the bubbles burst and don’t flow over the edges of the pot. I noticed some comments stating that although this is effective, oil coats your pasta and thus, does not allow for the sauce to be properly absorbed. Do you know of any other surfactant that would be food safe (FDA compliant) and could mitigate the foamy bubbles that appear due to the starch in the water? Any help would be greatly appreciated.
Kind regards,
Nelson
Nelson,
You can use Prospector Knowledge Center site to locate materials while reading all of my colleagues excellent articles. What you would be looking for would be a food grade defoamer. Years ago I worked for Colloids, which was then bought by now Solvay. We sold defoamers that were both kosher and of course foodgrade. These were for processing potato chips. Potatoes are notorious for foaming. I’m sure if you did a search on foodgrade defoamers, you would find ones specific to starches. Silicones work extremely well with starches and some are approved for use with food. If you’re just looking at home, there really isn’t anything wrong with using oil. I don’t really know that it would coat your pasta. If you boil the water, add the pasta, and then add the oil, there isn’t any way for the oil to coat the pasta. The oil would be at the surface. Even when you rinse, the pasta has seen water first, and then oil. So the amount that is left would be minimal.
Thanks for that very informative piece. Please what surfactant is known as P-77 surfactant? and what are the common alternatives around?
Thanks
P–77 is the designation for Pluronic, which is a block copolymer surfactant. There are several manufacturers of it, including BASF.
Marc, could you recommend someone point me I. The right direction to creating a detergent suitable for injection into water soluble film? I’m looking to create detergent pods. Any help would be great. Or if anyone can help me I would appreciate that as well.
Marvin, there are two parts to this problem, and neither one falls under anything obvious in Prospector Knowledge Center. The water-soluble film can be many things, but most likely cellulosic/PVP. You’re asking about detergents which are concentrated and there have been several webinars given by suppliers through UL. I suggest you look through those or contact supplier for guidance.
Hi Marc, I am looking for the best surfactant for aqueous waterproofing on wood and concrete surfaces with liquid sodium silicates and water. Any surfactant is best for good penetration and with a good forming of micro crystallisation?
Is this a trick question? Aqueous sodium silicate usually has a pH of 10 to 13 which is why it is never used on wood. I’ve never heard of anyone using sodium silicate or potassium silicate on anything except masonry. That being said, you would want a low viscosity surfactant that is tolerant to high pHs. I won’t recommend anything because I don’t have any idea where you are in the world. Contact your local global surfactant supplier and ask them.
Hi marc.l am looking for the best surfactant for water base carboxylated styrene butadiene latex.
Can you help me?
Okay so if I understand correctly, you are looking for a surfactant to emulsify carboxylated styrene butadiene. A lot will depend on what your Tg will be, which will be the basis of styrene versus butadiene in the latex. Generally, people use SLS and a whole host of favorite surfactants depending on what company you work for. I’m not sure what “best” is – best for what?
Hi sir,based on use I can suggest u water based antifoam
I approved this comment, although I don’t have an idea of what you are referring to. If you read other posts by me and by other people, we have posted other articles on defoamers and antifoams.
Dear sir,
Thank you for putting us through on this matter. I really appreciate.
Please what is the cause of orange in substrates after painting and how can it be avoided
Sir,
What I asked in my comment is about orange peel
Orange peel is caused by differential drying in a paint film. The most common causes are defoamer, viscosity and surfactants. If the defoamer is too strong; say a silicone, in a low viscosity coating, orange peel or fish eyes are apt to occur. The viscosity of 70 KU or thin film. Another cause could be what the coding is going over. I assume that there is orange peel no matter what the substrate is. There is never an easy answer in coating defects. I mention surfactant because orange peel is really something that is poorly dispersed in the coating or incompatible. I mentioned in one of the articles that care must be taken in how the coating is applied. Using a drawdown bar, you may see crawling, but there is very little sheer associated with that. If you were to spray the same coating, you would not see any defects. Like I said, there is no easy fix
Excellent work Marc.
Fred, thanks.
Impressive! Thanks for sharing this.
Hello Marc,
Aranya from India. A very Insightful article.
Recently, I am learning wax emulsion preparation.
Is it possible to make an stable wax emulsion only with anionic surfactants? I tried but failed. It is preferably stable in a non-inonic system.
Kindly share your thoughts on it. .
Aranya, I can suggest some things indirectly to you. First you can contact the supplier of wax emulsions and asked them for suggestions of what you are doing. I suggest you ask for several surfactants. No need to share any more information than that. The second is a little more tricky. You can use an amphoteric surfactant by itself or in conjunction with the noon-ionic surfactant. An amphoteric surfactant should compatibilize the system. The question is why do you need a wax emulsion with an anionic surfactant? I am guessing for compatibility with the resin. You can do a simple test of compatibility of resins with surfactants.
Hope this helps. Marc
Thanks Mark,
I’m working hard on removing surfactants back out of the environment using a process we’ve developed called surface active foam fractionation. We’re focussing it on PFAS compounds but it works of course on a range of surface active compounds such as the range you have so eloquently described. Thanks you for the description. It helped me better understand the chemistry behind what we are trying to remove from the environment.
Hi Marc, i do have a few very general questions regarding nonionic surfactants. Hope you provide some pointers to understand.
1. What pros or cons will a non ionic surfactant with a higher amide provide over a surfactant with a lower amide content? Generally speaking, higher amide content is better because it has more active surfactant matter?
2. How can we increase viscosity for a non ionic surfactant??
Ryan, all additives are trade-off. You may want higher amide, but you have water sensitivity issues as well as cloud point, which could be a problem with turbidity.
I don’t know that I’ve ever heard of anybody just wanting to increase the viscosity of the surfactant. You can reduce the temperature and if you want to make a surfactant slurry you can use HEC, Xanthan for or guar.