
CLEVELAND – Wearables technology is hot. In a society of information junkies, its purpose is to create constant, convenient, seamless, portable, and mostly hands-free access to information.
The global wearables market grew 223 percent in 2015’s second quarter (to 18.1 million units, up from 5.6 million in the same year-ago period), according to International Data Corp. It’s predicted to grow at a 45 percent compounded annual rate, and be an $80 billion market by 2020.
And while more than 90 percent of such devices are worn on the wrist today that, too, will evolve, as advanced technologies allow for the creation of new form factors.
Nowhere are these forces more at play than in the healthcare technology sector, where three trends are helping to drive growth – home-based healthcare, smartphone innovation, and flexible/printed electronics.
Enter John Gannon, president and CEO of Blue Spark Technologies Inc., who is sitting smack-dab in the middle of this evolution. Gannon’s firm began life in 2002 as Thin Battery Technologies, which was created to develop thin, flexible printed batteries built on intellectual property acquired from Eveready Battery Co. (now Energizer Holdings Inc.). Blue Spark today is based in Westlake, Ohio, just west of Cleveland and right across the street from Energizer’s global research center.
Looking for materials for your wearable technology design project?
Prospector has thousands of plastics materials from global suppliers, complete with technical data.
Search Plastics Materials

A former aerospace engineer and Merrill Lynch currency derivatives trader, Gannon in 2004 founded venture-capital firm SunBridge Partners, which funded some of Blue Spark’s early-stage development work. He then joined Blue Spark in his current leadership roles in fall 2011 (while remaining a partner at SunBridge).
Blue Spark already had done a lot of work developing a time temperature data logger for supply chain logistics using NFC, or Near Field Communication, wireless technology. And Gannon began to wonder what would happen if they stuck a time temperature logger onto something akin to a Band-Aid.
He now has the answer: TempTraq, the only single-use, wireless, wearable thermometer for kids in the form of a soft, comfortable patch. Using Bluetooth low-power technology, the patch continuously senses, records and sends alerts of a child’s temperature to your mobile device. It allows parents and caregivers the ability to monitor a sick child’s temperature even while the child sleeps.
But, like for anything that never existed before, the product development path included its share of bumps. Blue Spark teamed up with product design consultancy Smartshape Design Corp. in Cleveland, and together they tackled the challenges.
For the patch material itself, they opted to use a long-commercialized, multiweave polyethylene foam from 3M Co. That thin layer of foam encloses, top and bottom, the flexible, polyamide-based circuit containing the electronics and the battery. Working with a group in Switzerland, Blue Spark adopted a standard microprocessor to drive the circuit.

“The most difficult aspect proved to be the adhesive,” Gannon told an audience at the BioOhio 2015 annual conference, held Oct. 26 in Cleveland. Gannon was on a healthcare wearables panel with Smartshape Design founder and president Mike Maczuzak.
How materials interact with human skin is vitally important. Just ask Fitbit, the maker of a range of popular activity-tracking wristbands.
In early 2014, Fitbit recalled more than 1 million of its $130 Fitbit Force wristbands, after roughly 1.7 percent of its users complained of skin rashes after wearing the device. The firm blamed the skin irritations on either nickel, a common allergen that was found in trace amounts in the metal part of the tracker, or on the wristband’s adhesive.
The company acknowledged that tests had found the Force was causing allergic contact dermatitis, a condition that occurs when substances touching the skin cause irritation or a red, itchy rash reaction.
Now consider that at Blue Spark, they wanted to adhere a patch to the sensitive skin of an infant child. The stakes were high.
For the adhesive, Gannon explained in an interview after his presentation, “We started by using acrylics. But one of the problems with them is that they set up. So you put one on and wear it for 24 hours, and before you’re done, you’re peeling skin off. So we ended up moving toward a silicone gel-based solution. That is the current product.
“This device has to stand up to the FDA’s durability testing for printing,” Gannon noted. “So, if you’re printing [electronic circuitry] on foam, and then doing standard ISO testing for label durability, that becomes fairly challenging.”
So the development team had to come up with an innovative solution – and did.
“We print on the backside of a very thin layer of clear polyester and then we adhere that to a double-sided foam – thus the actual printed material is completely protected by a PET layer, rather than having it just sit on a PET layer that’s exposed.”
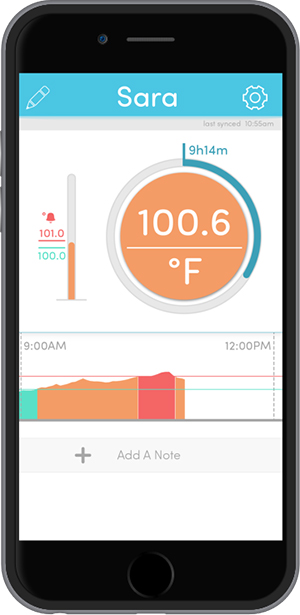 So, with the patch, the printed electronics and the adhesive issues resolved, Blue Spark had to fine-tune the wireless communications between patch and smartphone or tablet. That meant also developing software, and TempTraq today offers a free app that works both with iOS and Android devices.
So, with the patch, the printed electronics and the adhesive issues resolved, Blue Spark had to fine-tune the wireless communications between patch and smartphone or tablet. That meant also developing software, and TempTraq today offers a free app that works both with iOS and Android devices.
The app displays both real-time and historical temperature data transmitted from the patch in graphical or tabular view. It allows users to send recorded information via email. Through the app, parents also can keep track of when their child eats, drinks or takes medicine. And for those caring for multiple children, the TempTraq app allows them to monitor multiple thermometers simultaneously.
The patch takes a temperature reading every 10 seconds, stores the data, and then also communicates it out to an app-enabled smartphone or tablet.
As for the data transmission itself, Gannon said, “The original prototype of TempTraq was an NFC device,” drawing on the technology that Blue Spark was using successfully with its supply-chain monitoring device. But the problem with NFC technology in this type of application, he explained, is that “you need to be within 1 inch of the read. If you get within 1 inch of a sick, sleeping kid, you’ve woke him up and you didn’t accomplish the goal. So we did redesign TempTraq around Bluetooth, to get that read range.”
He says the patch now can deliver a signal to app-equipped smartphones or tablets within about a 40-foot range. One of the firm’s goals for 2016 is to develop a cloud-based software platform, to allow long-distance, remote monitoring.
TempTraq recently gained clearance as an FDA Class II medical device. The firm began production in May and has manufactured 80,000 units so far.
Meanwhile, Gannon said, Blue Spark also is working to develop longer-duration, 48- and 72-hour versions of the patch. The current, single-use, 24-hour patch retails for about $16-$19 each. Introduced at the 2015 Consumer Electronics Show in Las Vegas this past January, the device got a major promotional bump when it won a “Best of CES” award from among thousands of new-product introductions there.
“Working with Blue Spark to develop TempTraq was a great project for SmartShape for a number of reasons,” Maczuzak said. “Rather than just a product, we helped develop the entire user experience.”
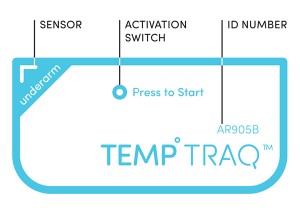 Since it was an entirely new type of product, “we needed to make it intuitively easy to understand – from reading and opening the packaging, properly applying the patch, downloading and using the app, adding additional children to monitor, changing to a new patch, etc.” SmartShape also helped to create the new TempTraq brand, from its logo to its packaging.
Since it was an entirely new type of product, “we needed to make it intuitively easy to understand – from reading and opening the packaging, properly applying the patch, downloading and using the app, adding additional children to monitor, changing to a new patch, etc.” SmartShape also helped to create the new TempTraq brand, from its logo to its packaging.
“Blue Spark is very knowledgeable about the thin-battery technology and about the suitable materials for the TempTraq patch – they’re the experts. This was a big advantage in getting to market quickly,” Maczuzak noted.
This past summer, Blue Spark began selling TempTraq online via Amazon, Sharper Image, Diapers.com, The FSA Store, Costco.com and CVS.com.
“Our goal is to get it into brick-and-mortar retail sites by late Q4 (2015) or early Q1 (2016),” Gannon said. And he also wants to introduce a fertility-tracking version next year. Due to the differences in each country’s regulatory rules, Gannon said they are focusing on the U.S. market alone for now, adding, “I don’t imagine us moving into Europe until late 2016.”
In the meantime, one can expect the feverish global market demand for medical wearables to continue.
The views, opinions and technical analyses presented here are those of the author or advertiser, and are not necessarily those of ULProspector.com or UL Solutions. The appearance of this content in the UL Prospector Knowledge Center does not constitute an endorsement by UL Solutions or its affiliates.
All content is subject to copyright and may not be reproduced without prior authorization from UL Solutions or the content author.
The content has been made available for informational and educational purposes only. While the editors of this site may verify the accuracy of its content from time to time, we assume no responsibility for errors made by the author, editorial staff or any other contributor.
UL Solutions does not make any representations or warranties with respect to the accuracy, applicability, fitness or completeness of the content. UL Solutions does not warrant the performance, effectiveness or applicability of sites listed or linked to in any content.


