The definition of dispersant, in terms of physical chemistry, is: a material that, when incorporated in to an admixture, is capable of homogeneously maintaining the dispersed particles in suspension. Therefore, the goal is to distribute dry pigments into a liquid medium and eliminate agglomerates.
Agglomerates are clumps of pigment, which, when not dispersed fully, affect many properties including¹ :
- Flocculation
- Flooding/floating
- Bénard cells
- Color shift/change
- Gloss decrease
Several types of additives can be used in the dispersion process in which solid particles, like pigments and fillers, are distributed and stabilized in a liquid. It is not enough to separate the particles, since they can re-agglomerate afterwards. This re-agglomeration is termed flocculation.
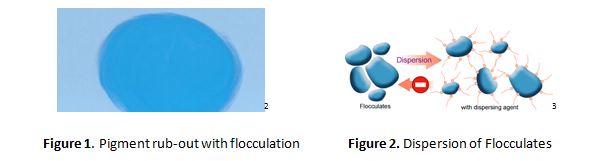
In Figure 1, a light blue paint has been applied to a substrate. Due to poor dispersion or flocculation, when the paint is partially dry, a test for dispersion is the application of shear by a finger in a circular motion (“rub-out test”). That energy in a semi-set paint film is sufficient to redistribute the pigment in the medium, resulting in the dark blue area. In addition to the dispersion of pigments, the surfactant displaces the air at the interface of the pigment particle. One of the more difficult materials to disperse has been single-walled carbon nanotubes, due to their morphology, and the strong attraction between the spindle-like particles which possess a high aspect ratio.
Looking for dispersants for your coatings formulations?
Prospector has hundreds of listings for disperant materials with technical data and the ability to request samples.
Search Dispersants
Dispersants absorb onto the pigment surface and therefore maintain pigment spacing by electrostatic repulsion and/or steric hindrance. Both stabilization mechanisms are described below.
Electrostatic Repulsion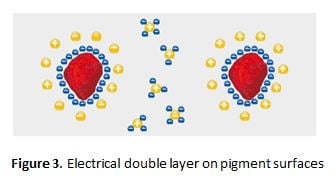
The pigment particles in the liquid paint carry electrical charges on their surfaces. Ideally, through the use of additives, it is possible to make all pigment particles equally charged. Counterions to the pigment-surface ions, concentrate at the pigment surfaces (in the liquid phase) so that an “electrical doublelayer” is formed (Figure 3). This is the ideal case and never wholly occurs practically.
The additives used for dispersion in such systems are polyelectrolytes – higher molecular weight moieties which contain a multitude of electrical charges in the side chains. The combination of side chains with a multitude of electrical charges, makes these chemicals useless as wetting agents.
Steric Stabilization/Hindrance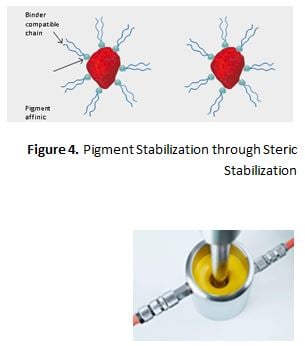
Dispersing additives, which function by steric hindrance, display two structural features. First, the products contain resin-compatible chains (hydro-carbon units) which, after adsorption of the additive onto the pigment surface, project into the surrounding resin solution. This layer of adsorbed additive molecules with the protruding chains is referred to as steric hindrance or “entropic stabilization” (Figure 4).
Second, these materials contain one or more groups that have a strong affinity to attach to the pigment, also known as “anchor groups” – that all together provide a strong, durable adsorption onto the pigment surface.
Steric stabilization occurs in solvent-borne systems and in water-reducible systems which contain solvated resins. Structural features composed of pigment affinic groups (polar) and resin-compatible chains (non-polar), these additives exhibit surface-active properties. Therefore, they not only stabilize the pigment dispersion, but they also function as wetting agents.
Author’s Note: In previous articles, topics like “Surfactants,” and “The Differences Between Wetting Agents and Dispersants” have been discussed. Here I solely present the subject of dispersants. If there is any confusion between the roles of wetting agents and dispersants, I suggest you read the latter article.
References:
[1] Coating Film Defects, Jochum Beetsma, UL Prospector 4/18/2014
[2] Dispersing & Wetting Hydrophobic Pigments & Fillers in Water-Based Paints to Avoid Pigment Flooding & Floating, Ron Lewarchik, UL Prospector 12/5/2014
[3] Dispersing Process, www.inkline.gr
The views, opinions and technical analyses presented here are those of the author or advertiser, and are not necessarily those of ULProspector.com or UL Solutions. The appearance of this content in the UL Prospector Knowledge Center does not constitute an endorsement by UL Solutions or its affiliates.
All content is subject to copyright and may not be reproduced without prior authorization from UL Solutions or the content author.
The content has been made available for informational and educational purposes only. While the editors of this site may verify the accuracy of its content from time to time, we assume no responsibility for errors made by the author, editorial staff or any other contributor.
UL Solutions does not make any representations or warranties with respect to the accuracy, applicability, fitness or completeness of the content. UL Solutions does not warrant the performance, effectiveness or applicability of sites listed or linked to in any content.



Buen articulo, es un tema, difícil de manejo en pinturas, la dispersión en mi concepto, maneja muchas propiedades y comportamiento de la pintura final.
Thank you. For those who don’t speak Spanish, my translation is not probably correct: “Good article, is an issue, difficult to manage in paints, dispersion in my opinion, manages many properties and behavior of the final paint.”