Properly chosen monomers and oligomers provide improved performance in a wide variety of coatings applications, such as:
- adhesion
- chemical resistance
- substrate wetting
- improved weathering
A monomer can be defined as a molecule that can combine with other molecules to form an oligomer or polymer. An oligomer can be described as a molecular complex comprised of a few monomer units. This article will focus on functional monomers and oligomer types that may be used in radiation cure compositions as well as solventless, high solids and waterborne coatings.
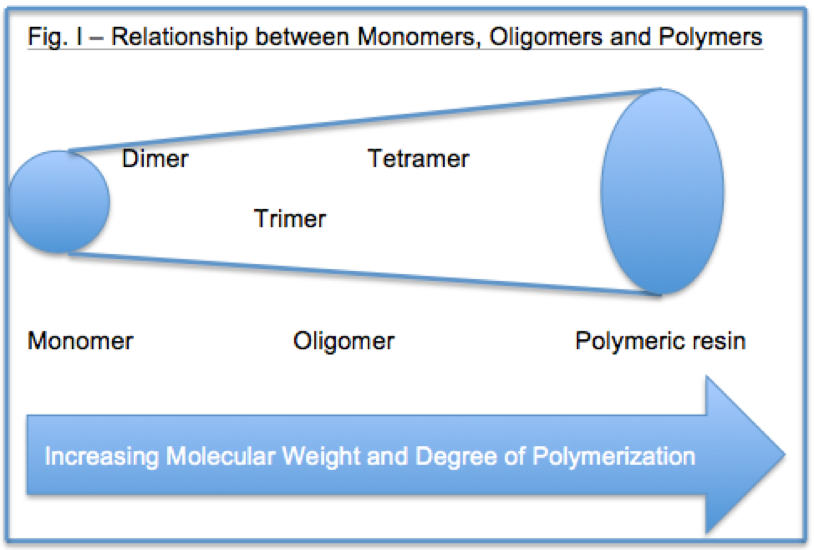
As the molecular weight increases in a solution polymer within a monomer type, viscosity increases, solubility decreases and in general compatibility with other resins decrease. Accordingly, with these basic relationships in mind, oligomers can be used to decrease volatile organic compound (VOC) content and application viscosity. Some of the common monomers and oligomers used in light cure systems include those in Figure 2.
Figure 2 – UV Cure Oligomer Types/Characteristics


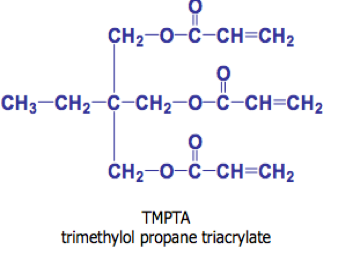

Table 1. Acrylate oligomer types, their performance effects and use in inks and coatings1
| Oligomer Type | Performance Effects | Use in Inks and Coatings |
| Epoxy Acrylate | Increase reactivity, hardness, chemical resistance; decrease cost | Oligomer of choice for coatings; used to lower cost in inks |
| Aliphatic urethane acrylate | Increase flexibility, toughness, weathering; multi-functionals increase reactivity, hardness, chemical resistance; decrease yellowing | Increase flexibility or hardness; for touch, weatherable screen inks |
| Aromatic urethane acrylate | Increase flexibility, toughness; multifunctionals increase reactivity, hardness, chemical resistance; decrease cost (vs. aliphatic) | Increase flexibility or hardness; not weatherable |
| Polyester acrylate | Increase wetting; decrease viscosity | For pigment wetting, adhesion; oligomer of choice for litho inks due to water balance and printability |
| Acrylic acrylate | Increase adhesion, weathering | Increase adhesion |
In addition to monomers and oligomers employed in radiation cure formulations, there is a myriad number of applications where these molecules are employed. For example, oligomers can be nonfunctional, mono functional or multifunctional and be used to enhance performance or environmental properties. Some common oligomer types used in applications in addition to light cure systems include epoxy ester, urethane, epoxy, acrylic, polyester and polycaprolactone. As figure III illustrates, many of these oligomers are functionalized with one or more functional groups to promote crosslinking, flexibility or adhesion.
Table 2. Examples of Oligomers Employed in Various Coating Applications
| Oligomer Type | Functional Groups | Potential Applications in Coatings |
| Silane | – SH, – Si-OH, -OH | Solvent free, multiple cures possible |
| Acrylic | – OH | 2K Urethanes, aminoplast cure |
| Brominated Epoxy | Oxirane | Flame retardant, cure with polyamines |
| Polyester urethane | – OH | Flexible topcoats, cure with –NCO, aminoplasts |
| Polycaprolactone | – OH | Cure with –NCO, aminoplasts |
In addition to the oligomer types described in Table 2, there are a host of other oligomers that are used primarily to reduce viscosity and VOC in coatings or to enhance flexibility, adhesion, weathering and substrate wetting.
As a class, the oligomers that are utilized to reduce viscosity in a formulation are commonly called reactive diluents. A reactive diluent performs two functions:
- to reduce viscosity similar to that of a solvent
- to react with the resin and cross-linker in the coating and thus become part of the cured coating film
The table below provides a number of examples of the types of functionalities that are available in oligomers that are classified as reactive diluents and the compositions they are used in.
Table 3. Examples of Functionalities in Oligomeric Reactive Diluents
| Functionality | Coating Application |
| Acrylate | Radiation cure |
| Vinyl | Radiation cure |
| Isocyanate | Urethanes |
| Oxirane | Epoxy based coatings |
| Mercaptan | Epoxy based coatings |
| Silane | Multiple resin types |
| Hydroxyl | Aminoplast or isocyanate |
| Acetoacetate | Crosslink with amino or isocyanate functionality |
Resources
- Radtech, Printers’ Guide: UV/EB Chemistry and Technology
- UL Prospector, UV-LED Cureable Coatings by Ronald Lewarchik, February 5, 2016
- Organic Coatings, Science and Technology, Third Addition, wicks et.al., Wiley Interscience, 2007
- UL Prospector raw material search engine
The views, opinions and technical analyses presented here are those of the author or advertiser, and are not necessarily those of ULProspector.com or UL Solutions. The appearance of this content in the UL Prospector Knowledge Center does not constitute an endorsement by UL Solutions or its affiliates.
All content is subject to copyright and may not be reproduced without prior authorization from UL Solutions or the content author.
The content has been made available for informational and educational purposes only. While the editors of this site may verify the accuracy of its content from time to time, we assume no responsibility for errors made by the author, editorial staff or any other contributor.
UL Solutions does not make any representations or warranties with respect to the accuracy, applicability, fitness or completeness of the content. UL Solutions does not warrant the performance, effectiveness or applicability of sites listed or linked to in any content.



That’s good to know that a monomer is a molecule that you can combine with other molecules. I probably had learned that in chemistry back in high school, but that was a long time ago and I totally forgot. That’s cool that people can build things using those monomers.
Dear sir ,
My name is Prashant
I am interested in development of radiation curable tack free top coat for fingernails
Chemical Dynamics provides product development services. In the event you are interested please contact us through http://www.Chemical Dynamics.net.