
Original article date: Aug. 13, 2020
Updated September 14, 2022
Most polyester resins used in coatings are functional resins and, as a class, normally lower molecular weight than thermoplastic polyesters used in the packaging and plastics industry, such as polyethylene (PE), polyethylene terephthalate (PET) or polybutylene terephthalate (PBT). Polyesters used in coatings applications are relatively low molecular weight and are amorphous, linear or branched and must be crosslinked to form useful films. As a class, thermosetting polyesters generally provide better metal adhesion, flexibility and thus impact resistance than thermosetting acrylics. However, the presence of ester linkages in the backbone of polyesters make them more prone to hydrolysis, accordingly proper selection of backbone monomers that provide steric hindrance to the ester group linkage, for example, NPG provides improved resistance to hydrolysis and weather resistance.
Polyester Synthesis
Historically, polyester synthesis was referred to as condensation polymerization as the reaction of an alcohol group and a carboxyl group produces water.
Polyester synthesis routes include:
- Step-growth polymerization
- The reaction of an ester with an alcohol
- The reaction of an anhydride and an alcohol
- Ring-opening polymerization of a lactone
Functional polyesters
Functional polyesters are available with a number of functionalities that include hydroxyl, carboxyl, carbamate, reactive unsaturation and a combination of one or more of these functionalities. By and large, most functional polyesters are hydroxyl functional polymers. Functional polyesters are very versatile as they can be engineered to provide excellent properties, including mechanical, impact and flexibility, hardness, UV and chemical resistance. Applications for functional polyesters include automotive, aerospace, military, industrial, coil coating, and construction and include conventional solids, high solids, waterborne and powder coating. Formation of polyesters is commonly accomplished by step-growth polymerization of an alcohol with at least two hydroxy groups and a carboxylic acid with at least two carboxyl groups.
Conventional solids polyesters
Most polyester resins for conventional solids coatings comprise a diol and triol coupled with an aromatic dicarboxylic and aliphatic dicarboxylic acid or anhydride. The mole equivalency of dibasic acid to hydroxy polyol must be under one in order to provide a polyester resin with terminal hydroxyl groups and avoid a gel. If an excess of dibasic acid is used, the polyester is carboxy terminated for reaction with epoxy, melamine or 2-hydroxyalkyl amides. The ratio of linear aliphatic to aromatic dibasic acid controls the Tg along with the selection of polyols effects the crosslink density, reactivity and the tendency of the resin to crystallize.
When a diol (DD) reacts with a dibasic acid (CC) in equal molar amounts, the molecular weight builds gradually and is more readily controlled. The reactant in excess will have terminal groups of that reactant. For example:
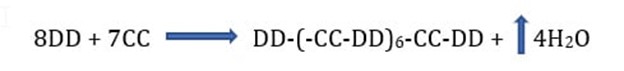
The average molecule will have terminal hydroxyl groups. Branched polyesters are made from mixtures of monomers that contain one or more monomers which have a functionality F > 2. As the proportion of a monomer with F (functionality) > 2 increases, the number average molecular weight increases and the reaction must be controlled to avoid gelation. A wide range of polyesters are in commercial use; for conventional polyesters cured with melamine or isocyanate prepolymers, the number average molecular weight is in the 2,000 to 6,000 range.
Resins for conventional solids polyester coatings have a low level of carboxyl functionality coupled with a higher level of hydroxyl functionality to formulate a coating that crosslinks with a melamine crosslinker or blocked isocyanate for single component thermosetting baked coating. Alternatively, an isocyanate functional prepolymer can be used for two-component ambient cure urethane formulations. Examples of the use of conventional solids hydroxyl-functional polyesters include industrial and aerospace paints.
Hydroxyl-terminated linear polyester
Most polyester polyols for coil coatings are hydroxyl-terminated linear polyesters and are preferred to maximize flexibility. Painted coils are fabricated after painting rather than painted before the metal is formed. Accordingly, the coil coating process is often called prepaint. Most coil polyester resins have little or no trifunctional hydroxyl building blocks (for example, TMP) and utilize diols such as neopentyl glycol, propylene glycol, butane diol or 1,6 hexane diol. The diacids used in these types of resins can be a combination of linear aliphatic diacids along with an aromatic diacid or their dianhydride. Depending on their application, coil coatings are rapidly cured at high temperatures and formulated with an aminoplast crosslinker such as melamine or a blocked isocyanate.
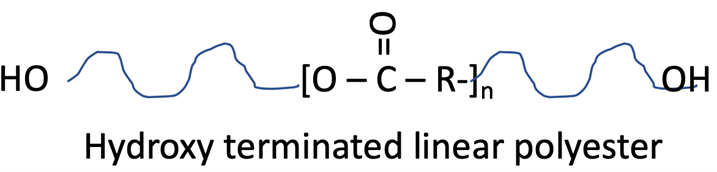
 The selection of polyester building blocks affects the architecture of the polyester molecule and many of the performance attributes such as flexibility, hardness, corrosion resistance, hydrolytic stability, exterior weatherability, chemical resistance, stain resistance and cure speed.
The selection of polyester building blocks affects the architecture of the polyester molecule and many of the performance attributes such as flexibility, hardness, corrosion resistance, hydrolytic stability, exterior weatherability, chemical resistance, stain resistance and cure speed.
Carboxy-functional polyester
Carboxylic acid terminated functional polyesters most commonly contain TMA (see Table II) and IPA. Such carboxylic acid functional polyesters can be crosslinked with melamine or, more commonly, epoxy functional crosslinkers. For powder coating applications, higher Tg carboxyl functional polyesters are based on TPA and IPA. Many so-called superpolyesters utilize IPA, NPG and other building blocks and can provide excellent hydrophobicity and light stability. Triglycidyl isocyanurate (oxirane) functional crosslinkers coupled with light and hydrolytic stable carboxylic functional polyesters provide very weatherable powder coatings.

Electrostatic spray application of powder paint on metal objects
Carbamate functional polyester
These polyesters can be crosslinked with melamine to provide coatings with good light stability, acid resistance and mar resistance.
Unsaturated functional polyester
These polyesters generally contain maleic anhydride or fumaric acid as the source of their unsaturation, along with suitable acid functional and hydroxy functional building blocks and styrene. Although these resins are most known for their application in gel coats, they are sometimes formulated with peroxide and cobalt initiators in a two-component formulation primarily for gel coat applications.
Table I – Effect of polyols on Polymer Properties:
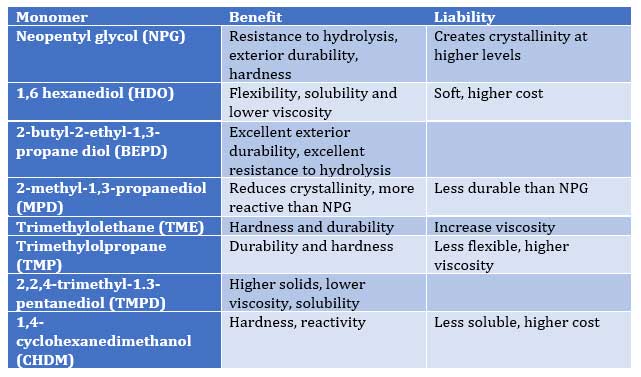
Table II – Effect of acid functional monomers on Polymer Properties:
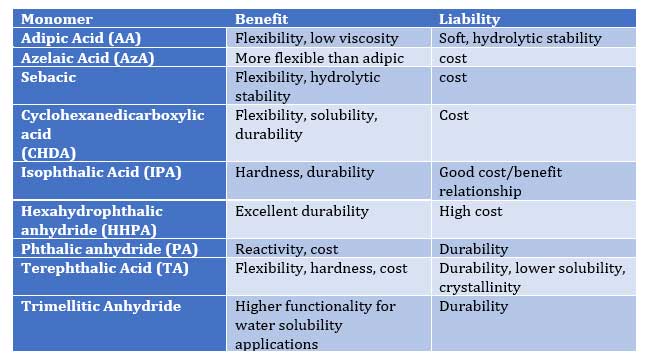
As Tables I and II illustrate, proper selection of co-reactant monomers can provide a range of performance characteristics to provide an array of performance attributes such as
- hydrolytic stability (NPG, Sebacic, CHDA)
- exterior weathering (NPG, BEPD, TMP, TME, HHPA, IPA)
- hardness (NPG, TME, TME, CHDM, TA)
- flexibility (AA, AzA, Seb, CHDA, TA, CDO)
Desired performance can be achieved through the proper selection of a blend of monomers coupled with the selection of the polymer architecture to meet film performance properties.
Resources
- Organic Coatings, Science and Technology, Frank N. Jones et al., Wiley & Sons, 2017
- Prospector Knowledge Center
- Prospector Raw Materials for coatings
The views, opinions and technical analyses presented here are those of the author or advertiser, and are not necessarily those of ULProspector.com or UL Solutions. The appearance of this content in the UL Prospector Knowledge Center does not constitute an endorsement by UL Solutions or its affiliates.
All content is subject to copyright and may not be reproduced without prior authorization from UL Solutions or the content author.
The content has been made available for informational and educational purposes only. While the editors of this site may verify the accuracy of its content from time to time, we assume no responsibility for errors made by the author, editorial staff or any other contributor.
UL Solutions does not make any representations or warranties with respect to the accuracy, applicability, fitness or completeness of the content. UL Solutions does not warrant the performance, effectiveness or applicability of sites listed or linked to in any content.



Very interesting theme, the different properties between monomers and their applications
Good article on basic polyester resin chemistry.
Would like to connect with the writer.
Good day Surendra! You can contact Ron at [email protected].
Ron,
I liked your article “Fundamentals of Polyester Resins” posted August 13, 2020.
It is a great place to introduce Polyester Resin chemistry.
Isn’t it true that equation below at 100% completion should yield 14 mole H2O?
8DD + 7CC–> DD-(-CC-DD)6-CC-DD + 14 H2O
Where can I obtain “2,2,4-Trimethyl-1,5-pentanediol”?
Thank you.